Park in Phoenix, bake your
airplane in the sun on the tarmac for a few hours (or days), depart late
afternoon, and then climb. At some point the already hot fuel, when further
heated on its way to the engine, is going to boil and you will not be a happy
camper. If you have not experienced it, it just means you have not truly heat
soaked your fuel and then climbed high enough.
I did just this during an ugly period
when I had to commute to Phoenix in my TR-182, a high
wing airplane. I sometimes had engine surging as I climbed to 12,500, probably
due to boiling of fuel in the carburettor bowl.. I descended until it stopped,
waited for the tanks to cool, and then resumed the climb to cooler air (my
ultimate goal) after about half an hour. With a fuel injected low wing
airplane, the problem would have been boiling on the way to the engine-driven
fuel pump, and the boost pump would have solved it.
The ultimate solution? Fuel pumps in the
tanks. But boost pumps are fine as long as you recognize what is going on
when you visit Phoenix in July
The following are extracted from the
Chevron aviation fuel web site.
http://www.chevron.com/products/prodserv/fuels/bulletin/aviationfuel/8_ag_perf.shtm
Fred
Vapor
Pressure
The vapor
pressure of a pure compound is defined as the pressure exerted by
its vapor in equilibrium with the liquid at a constant temperature. This
pressure is independent of the amount evaporated, or vapor-liquid ratio, as
long as there is liquid remaining. Vapor pressure increases as temperature
increases. When the vapor pressure is equal to the external pressure (usually
atmospheric pressure), the liquid boils.
But fuels, which are mixtures of many different
hydrocarbons, do not behave the same as pure compounds. Each individual
component in the fuel has its own vapor pressure. The vapor phase over a fuel
does not have the same composition as the liquid phase. The vapor phase
contains more of the high vapor pressure (low boiling point) compounds than the
liquid.
As a fuel evaporates, its composition changes. The
vapor pressure of the remaining liquid decreases as the extent of evaporation
increases. For a complex mixture such as a fuel, vapor pressure is defined as
the pressure of the vapor in equilibrium with its liquid at a specified
temperature, as the vapor to liquid ratio approaches zero. This is the highest
pressure that a fuel can exert.
In the petroleum industry, the true vapor
pressure as defined above is difficult to measure in practice, and is therefore
not often used. Instead, a related quantity called the Reid vapor pressure (RVP) is measured.
The RVP of a fuel is typically a few percentage points lower than the true vapor
pressure because of the way it is measured.
Elsewhere:
Fuel Boil-Off
The boiling point of a liquid is the temperature at which its
vapor pressure is equal to the local atmospheric pressure. However, atmospheric
pressure decreases exponentially with increasing altitude. At 3000 meters
(10,000 feet), atmospheric pressure is only about 69 percent as great as at sea
level, and at 6000 meters (20,000 feet), it drops to 46 percent of the
sea-level value. Thus liquids boil at lower temperatures at higher altitudes.
Atmospheric temperature also decreases as altitude increases,
which tends to counteract the decrease in pressure. However, it takes time for
fuel to cool to ambient air temperature, while pressure equilibration is almost
instantaneous since fuel tanks are vented to the atmosphere. An aircraft taking
off from sea level with a fuel temperature of 38ºC (100ºF) and ascending
rapidly to 6000 meters (20,000 feet) may experience fuel vapor pressure that
is greater than the pressure in the fuel tank, i.e., the fuel will boil. [Emphasis added.] Any boil-off
that does occur will likely be confined to the fuel tank since fuel pumps
maintain the rest of the fuel system at a higher pressure. [But it can
occur in the supply lines leading up to the fuel pump, particularly if the
pressure is lower in the supply line, as in sucking it up hill, and if the fuel
is further heated.]
If fuel does boil, the components with the highest vapor
pressure evaporate first. Loss of these lightest components changes the
composition of the remaining liquid and leaves it with a lower vapor pressure.
When the fuel vapor pressure drops below the ambient pressure in the tank,
boiling will stop. Fuel boiling also lowers the temperature of the remaining
liquid through evaporative cooling. Both of these effects tend to minimize loss
of fuel due to boiling. Only in extreme circumstances will loss of fuel exceed
a few percent.
The solubility of air dissolved in fuel also decreases as
pressure decreases. Normally this air will come out of solution smoothly and
not cause a problem. However, if the fuel becomes supersaturated, air can be
evolved very quickly and cause frothing of the fuel. This can lead to loss of
fuel from vents, but the mechanism is different from actual fuel boiling.
Vapor Lock
Vapor lock
occurs when excessive gasoline vapor accumulates somewhere in the fuel system
– fuel pump, fuel line, carburetor or fuel injector – and reduces
or interrupts the fuel supply to the engine. When the fuel supply is reduced,
the fuel-air ratio becomes too lean, which may result in loss of power,
knocking, surging, or backfiring. When the fuel supply is interrupted, the
engine stops and may be difficult to restart until the fuel system has cooled
and the vapor recondensed, or purged by boosting the fuel supply pressure.
While the tendency of avgas to vapor lock increases with
volatility, fuel overheating is the main cause of vapor lock. Local
temperatures in the fuel system are determined by how hard the engine is
working and how well the fuel system is isolated from the heat of the engine.
Fuel residence time in the hot sections of the system, mechanical vibration,
and other factors also play a significant role in vapor lock behavior.
The altitude at which the engine is operating has two
opposing influences: ambient temperatures are lower at higher altitudes, which
should improve fuel system cooling; but ambient pressures are also lower,
making vaporization easier.
The design of an aircraft fuel system must take all the above
factors into account to ensure that liquid fuel, with little or no free vapor,
is delivered to the engine's fuel metering system.
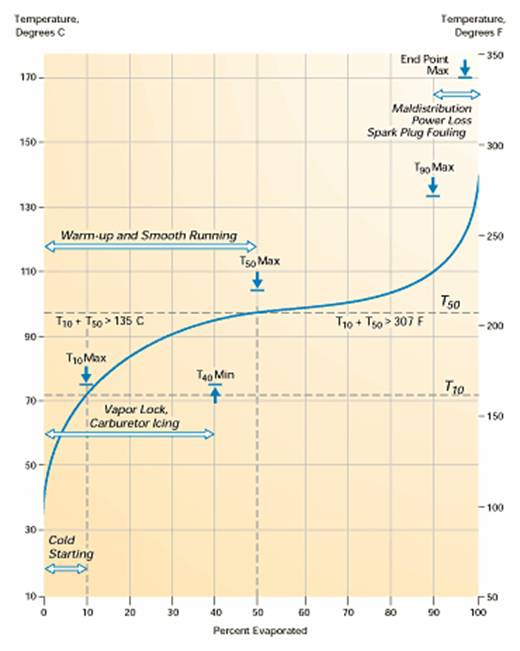
Below is a typical distillation curve for avgas.